Five Prime Therapeutics, Inc., a clinical-stage biotechnology company, focuses on the discovery and development of innovative protein therapeutics. The company's product candidates comprise Bemarituzumab, an antibody that inhibits fibroblast growth factor receptor 2b, or FGFR2b, which is in Phase 3 clinical trials to treat patients with gastric or gastroesophageal junction and GEJ cancer; and FPA150, a CD8 T cell checkpoint inhibitor antibody that is in Phase 1a/1b clinical trial that targets B7-H4 in various cancers, as well as FPT155, a soluble CD80 fusion protein, which is in Phase 1a/1b clinical trial that enhances co-stimulation of T cells through CD28. Its product candidates also include Cabiralizumab, an antibody that inhibits colony stimulating factor-1 receptor that is in Phase Ia/Ib clinical trials for the treatment of various cancers in combination with Opdivo. The company's BMS-986258, an anti-T cell immunoglobulin and mucin domain-3 antibody, which is in Phase 1/2 clinical trial as a single agent and in combination with Opdivo in patients with advanced malignant tumors. It has license and collaboration agreements with Bristol-Myers Squibb Company, GlaxoSmithKline LLC, INBRX 110 LP, UCB Pharma S.A., and Zai Lab (Shanghai) Co., Ltd.; and license agreements with Galaxy Biotech, LLC, BioWa, Inc. and Lonza Sales AG. The company was founded in 2001 and is headquartered in South San Francisco, California.
![[Bild: FPRXc0ml1038.png]](https://www.finviz.com/publish/111120/FPRXc0ml1038.png)
![[Bild: FPRXc0wl1038.png]](https://www.finviz.com/publish/111120/FPRXc0wl1038.png)
![[Bild: chart.ashx?t=FPRX&ty=c&ta=0&p=d&s=l]](https://www.finviz.com/chart.ashx?t=FPRX&ty=c&ta=0&p=d&s=l)
![[Bild: FPRXc0ml1038.png]](https://www.finviz.com/publish/111120/FPRXc0ml1038.png)
![[Bild: FPRXc0wl1038.png]](https://www.finviz.com/publish/111120/FPRXc0wl1038.png)
__________________

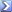
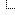
 offline
offline
